Clinical Trial Results Published in Scientific Reports
— Seeking U.S. Partners for Market Entry —
KYOTO, JAPAN — [February 13, 2025] —Sanyo Chemical’s Silk-Elastin, a novel regenerative medicine material, has demonstrated promising results in clinical trials for meniscus regeneration. Findings published in Scientific Reports validate its safety and efficacy, highlighting its potential as a groundbreaking regenerative treatment for meniscus injuries and paving the way for FDA approval and commercialization in the U.S. To bring this innovative meniscus repair treatment to market, Sanyo Chemical is seeking marketing and distribution partners in the U.S.
“We are very pleased to have our clinical trial data published in such a prestigious scientific journal,” said Akihito Higuchi, President & CEO of Sanyo Chemical. “Meniscus injuries affect many people, often with limited treatment options, and we are committed to bringing this breakthrough treatment to patients as soon as possible. To achieve this, we are eager to partner with global companies that share our vision for advancing regenerative medicine.”
A New Approach to Meniscus Injury Treatment
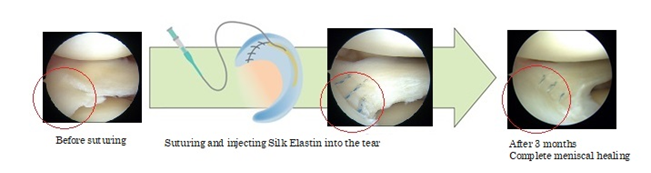
Meniscus injuries, common in sports and aging, are often treated with meniscectomy (partial or full removal), which increases osteoarthritis risk. Silk-Elastin offers a regenerative alternative, promoting repair rather than removal, potentially preserving joint function.
Clinical Applications and Development Status
Research with Hiroshima University confirms that Silk-Elastin facilitates meniscus repair and regeneration, potentially reducing the need for surgical removal. Promising investigator-initiated trials in Japan have led to corporate clinical trials beginning summer 2025.
Published Study in Scientific Reports
- Title: First-in-human exploratory trial assessing safety, feasibility, and efficacy of artificial protein (silk-elastin) in promoting healing in patients with meniscus injuries
- Authors: Masakazu Ishikawa, Nobuo Adachi, et al.
- Publication Date: 07 February 2025
- Summary: This first human trial of Silk-Elastin demonstrated its safety, feasibility, and efficacy in promoting meniscal healing. Patients showed improved tissue integration and recovery, positioning Silk-Elastin as a viable alternative to meniscus removal surgery.
- Reference: https://www.nature.com/articles/s41598-025-88616-x
About Silk-Elastin
Silk-Elastin is a recombinant protein combining the strength of silk fibroin (derived from silk) with the elasticity and biocompatibility of elastin (found in human skin). Its gel supports tissue regeneration while minimizing inflammation. This novel approach offers hope for conditions with limited conventional options.
About Sanyo Chemical
Sanyo Chemical established in 1949 in Kyoto, Japan, is a global manufacturer and seller of performance chemicals. Beginning as a manufacturer of soap and texture agents we have since diversified our product portfolio to meet the needs of the market, Today, we feature over 3,000 diverse types of products and have established an international presence. Our portfolio of chemicals spans a variety of industries and types, from automotive components to daily-use electronics, as well as cosmetics and medical equipment, all with the aim of creating safe and environmentally friendlier offerings, improving lives and societies across the world. We aim to contribute to realize a sustainable society through our corporate activities
https://www.sanyo-chemical.co.jp/eng
Contact:
For business inquiries:
Sanyo Chemical Industries, Ltd.
Siela Project
Phone: +81-75-541-6249
E-mail:pr-group@sanyo-chemical.group