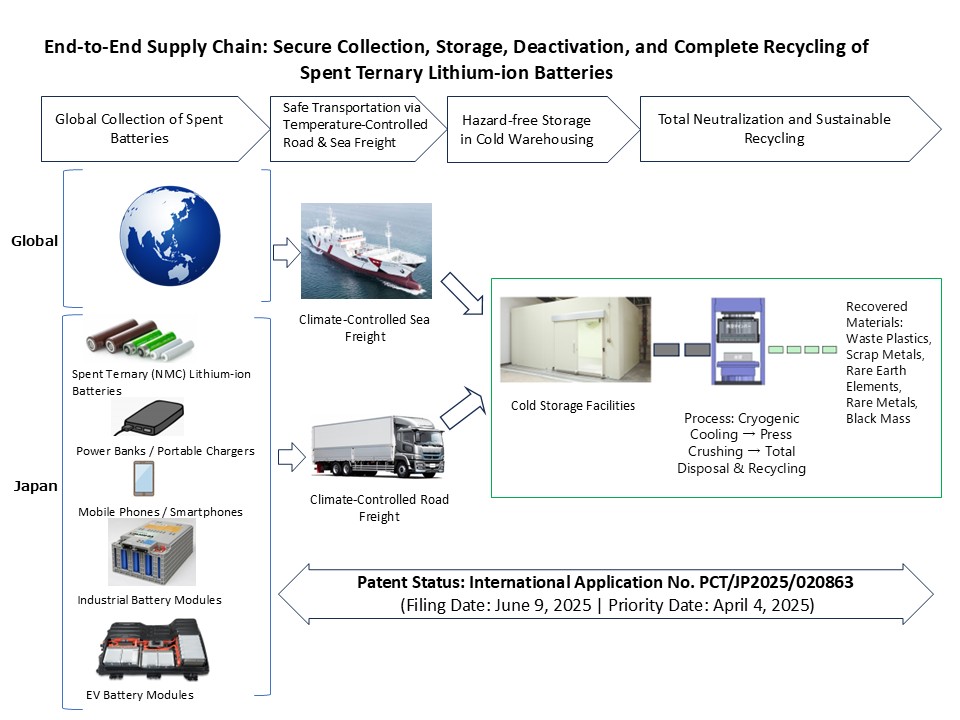
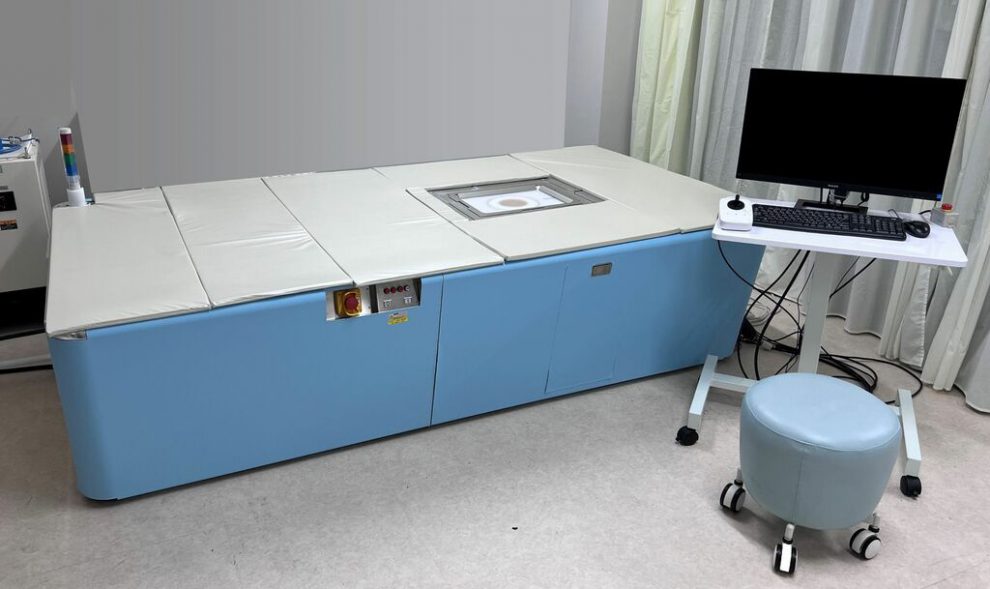
Radiation-free, non-invasive system visualizes the structure and function of the microvascular network
Luxonus Inc. (Kawasaki, Kanagawa, Japan, Sadakazu Aiso, President & CEO) announced that its application for a medical device marketing license for its photoacoustic 3D imaging system has been approved by Japan’s Pharmaceuticals and Medical Devices Agency (PMDA).
Luxonus will begin full-scale sales promotion in Japan while simultaneously taking steps to enter international markets by starting preparation to obtain medical device approvals in various countries.
<Photoacoustic 3D Imaging System>
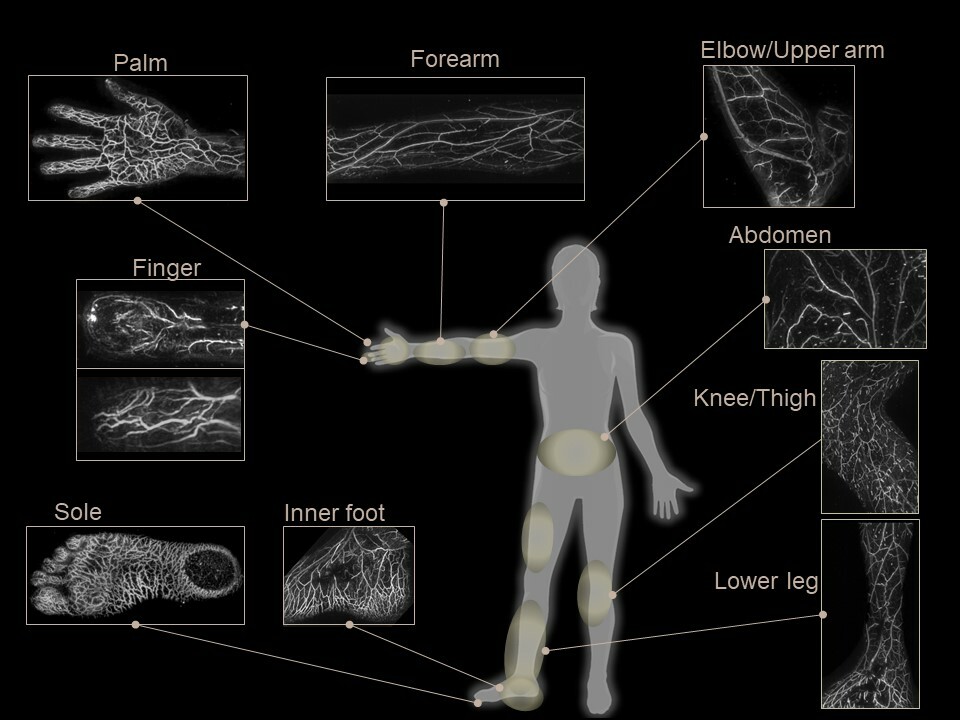
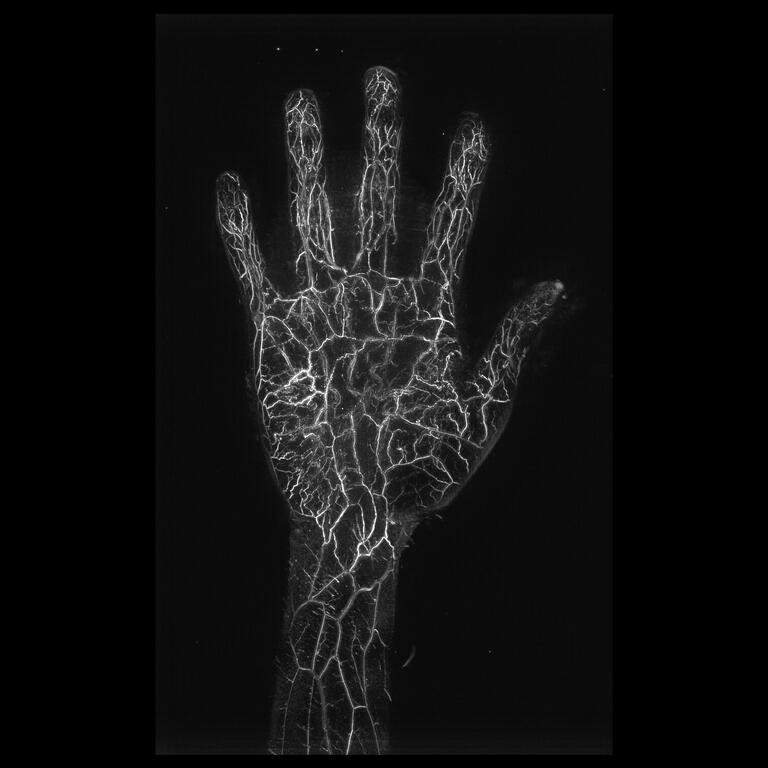
The photoacoustic imaging system is an unprecedented diagnostic device that evaluates the morphological state of blood flow in 3D without exposure to radiation and without the use of contrast media. By irradiating near-infrared pulsed laser light onto the body surface, ultrasound waves are generated by hemoglobin in the blood then detected by a unique ultrasound sensor array, enabling three-dimensional visualization of minute blood vessels as small as 0.2 mm.
The system has already been released in Japan for use on laboratory animals.
Luxonus’ mission is to provide solutions to various unmet medical needs, including the early diagnosis of vascular abnormalities, by utilizing photoacoustic 3D imaging to render three-dimensional, high-resolution images of blood vessels.
Installation and use of the photoacoustic 3D imaging system eliminates the risk of exposing patients and medical personnel to radiation, thereby limiting liability, eliminating the need for diagnostic facilities to be designed for radiation exposure mitigation, and providing peace of mind. In addition, its automatic operation makes it possible to obtain objective and reproducible images easily and quickly, regardless of operator technique.
<Luxonus>
Luxonus is a start-up company established in 2018 to commercialize photoacoustic imaging technologies developed under the Cabinet Office’s Impulsing Paradigm Change through Disruptive Technologies Program (ImPACT).
This development is supported by the Japan Agency for Medical Research and Development (AMED) and the New Energy and Industrial Technology Development Organization (NEDO).
<Contact>
address: AIRBIC A22, 7-7 Shinkawasaki, Saiwai-ku, Kawasaki City, Kanagawa, Japan, 212-0032
e mail: lux-info@luxonus.jp
URL: https://www.luxonus.jp/toppageen/
Luxonus Inc.
email: lux-info@luxonus.jp
URL: https://www.luxonus.jp/toppageen/
Linkedin; https://www.linkedin.com/company/luxonus/